Calcium hydroxide is the chemical name for hydrated lime. It is traditionally referred to as slaked lime. Hydrated lime is a colorless crystal or white powder and is obtained once burnt lime (called lime or quicklime) is mixed, or quenched with water. Hydrated lime is also known as caustic lime, builder’s lime or pickling lime. It is employed in several applications, as well as food preparation.
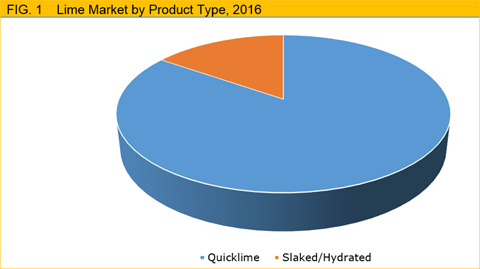
In the global market, lime products are primarily categorized into quicklime and hydrated lime. Quicklime, the starting material in manufacturing hydrated lime, occupies larger market share of about 84.7% as compared to hydrated lime due to its versatility in applications.
Figure 1: Pie Chart showing the sales of lime products in global markets in 2016.
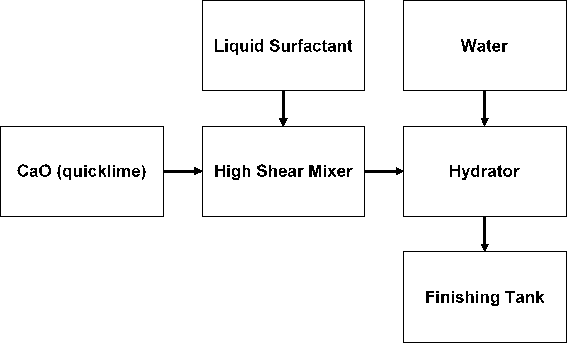
Synthesis Process:
Quicklime (calcium oxide) is added into an industrial mixer. Liquid surfactant with no water content is then added into the mixer to coat the surface of quicklime to ensure that is no moisture in the mixture. Liquid surfactant is added in batches continuously until all quicklime are coated.
Stoichiometric amount of water is then added to the mixture in a hydrator at atmospheric pressure. The hydrated lime material is then transferred to a finisher to ensure complete hydration takes place.
Hydrated lime can also be synthesized from high dolomitic lime and high calcium lime.
Enzymes like cationic and anionic species are often added to facilitate coating and hydration.
Hydrated lime has many applications.
Paper industry:
Iit is used as the intermediate in the reaction to produce sodium hydroxide. This is the causticizing step in the Kraft Process for making pulp.
Water and Sewage Treatment Plant:
It is mainly used as flocculant in water and sewage treatment. It precipitates with impurities in the water, forming a positively charged solid.
Food industry:
Hydrated lime is used in the carbonatation processing in purifying raw juices from sugarcane and sugar beets. It reacts with the carbon dioxide in the raw juices and precipitates as calcium carbonate. Hydrated lime is also used in the preparation of pickled food and processing of water in carbonated drinks.
Since it is the key ingredient in carbonatation process, it is also used to remove carbon dioxide from controlled atmosphere produce storage room.